The Science behind COVID-19 Testing
Prof. Jason Chan, Assistant Professor of Science Education in the Department of Chemistry, brings us lesson to the science behind COVID-19 Testing.
By Prof. Jason Chan, Assistant Professor of Science Education in the Department of Chemistry
Resurgence of COVID-19 cases in Hong Kong once again left us all worrying about our safety in the midst of the global pandemic. Laboratory tests for the coronavirus is our essential frontline defence to identify infected persons for treatments and isolation. In this article, let’s take a look at the science behind how testing for the novel coronavirus is carried out.
To receive testing, a deep throat saliva sample will be collected and sent to the laboratory for screening to see whether it contained the SARS-CoV-2 virus. The screening test is based on One-step Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR). To appreciate how the test works, we need to break it down into smaller parts.
1. Reverse Transcription (RT)
Our genetic information is stored and encoded in the sequence of four bases (A, T, C, G) on a polymer chain called DNA (deoxyribonucleic acid). Different stretches of these bases would encode for amino acid sequences of our proteins.
When our body needs to manufacture a certain protein, it would start by making a copy of the genetic codes to use as the transient storage. The cell chooses to write this transient copy in RNA (ribonucleic acid). The RNA copy of the gene is known as messenger RNA (mRNA). This process of forming mRNA from the DNA is known as transcription.
RNA viruses use RNA instead of DNA to store their genetic information. Some of them use their viral RNA directly as mRNA inside a host cell(such as SARS-CoV-2), while others (such as HIV viruses) convert their RNA into DNA once they enter a host cell to allow them to insert their viral sequences into the host’s DNA genome and trick the host to make copies of the virus. This process of making a DNA copy from RNA is the reversal of transcription, known as reverse transcription. RNA viruses have an enzyme called reverse transcriptase that carries out this reaction and the DNA produced from the RNA in this way is called complementary DNA (cDNA). cDNA is needed for the next step, PCR, as the template material.

2. Polymerase Chain Reaction (PCR)
The PCR reaction produces millions of copies of a target segment of DNA from even a single copy. This amplification of DNA sequence can provide scientists with sufficient amounts of DNA materials allowing them to study and work with them, such as to check if a match is present with a viral sequence.
The PCR reaction mixture contains at least these key components:
- a template (the original DNA material from which copies are made)
- a heat-stable DNA polymerase (an enzyme to make DNA copies)
- two short DNA pieces called primers (to mark the start and end of the target section)
- the substrates for making new DNA chains (dNTPs)
- a magnesium salt (Mg2+ is a co-factor for the polymerase)
- buffers (to maintain optimal pH for the enzyme).
This mixture is placed into a small plastic tube and into a thermocycler that would subject the mixture to cycles of the two key temperatures: 95 oC (3 sec) and 55 oC (30 sec).
At 95 oC, the template, in our case, the double-stranded cDNA from reverse transcription step would separate into two single strands. This step is called denaturation.
Then upon cooling to 55 oC, two DNA primers which are specific to SARS-CoV-2 sequences would now try and find complementary sequence to pair up with. If the cDNA from the virus is present, they will find the sequence and bind with it. This step is called annealing. Once the primers annealed with the correct sequence, DNA polymerase enzymes would also build new DNA copies during this period. This step is called extension. After this step, we would end up with two DNA strands from only one that was started with.
When the temperature is returned to 95 oC for the next cycle, the extension would stop as the newly formed double-stranded DNAs separate into single strands. They will be ready to act as templates when they hit 55 oC.
With each cycle being repeated, the number of DNA strands present would be doubled, such that after 45 cycles, there will be up to 245 copies of the target the DNA present.

*Table shows the screening test reaction condition. Note that the UNG incubation step is an error prevention step to eliminate cross-contamination between samples. (CDC Protocol)
3. Real-time or Quantitative PCR (qPCR)
One inconvenience for the usual form of PCR is there’s no way to know if the reactions are working well until all cycles are completed and you run a test to check the products. Scientists developed a modified version of PCR that allowed them to monitor the PCR reaction in real time as copies are being made. This is called real-time PCR or quantitative PCR. This is achieved by adding a DNA probe that has complementary sequence to a short region within the target gene. This probe contains two features: a reporter fluorescent dye (FAM) capping one end and a quencher unit (BHQ1) at the other end. When both ends are attached together on the probe, the quencher prevents the fluorescent dye from glowing and giving any signals.
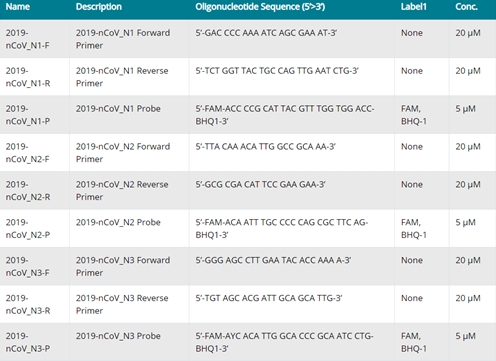
* Forward and reverse primers and probes used for screening tests. (CDC Protocol)
During the annealing step, the probe would bind onto the template strand and the polymerase would eventually reach that position during chain extension. At this point, the polymerase would cleave off the fluorescent reporter from the probe, and fluorescence can be observed under UV light. Measuring the intensity of fluorescence signal gives us a way to tell if the PCR is going well within the tubes.

*Diagram illustrating how the real-time probe works. Blue circle = fluorescent reporter (FAM or 6-carboxyfluorescein) Red circle = quencher Yellow = DNA polymerase
4. One-step RT-qPCR
Mucus samples collected from the deep throat of patients suspected of infection may contain the coronavirus and the viral RNA can be detected by a RT-qPCR reaction.
The first step is to convert the RNA in the viruses into cDNA by reverse transcription. The cDNA is then used for qPCR reaction in the next step.
Traditionally, one would have to extract the cDNA from reverse transcription (RT) before adding that into the qPCR reaction mixture. This two-step procedure is slow and tedious. In one-step RT-qPCR, these two steps have been combined into a one-pot reaction, which increased efficiency greatly. Temperature alone can control RT or qPCR steps.

*One-step VS two-step RT-qPCR
The test result would be considered positive if fluorescence signals from qPCR was detected to be above a set threshold level.

*Fluorescence signal in qPCR measured against the number of PCR cycles. Red line indicates the threshold level. (CDC Protocol)
In this article, we described the original protocol developed by CDC. Improved procedures have since been developed that would provide higher sensitivity (e.g. SYBR® Green probes).
5. Control experiments
The reliability of COVID-19 tests is of paramount importance. After all, no one would want to be falsely diagnosed with it (called false positive). On the other hand, it would pose a great danger to the public if the infected were mis-diagnosed as negative (called false-negative).
RT-qPCR tests are not fail-proof and it is possible to mess up at the various stages of the test. To prevent errors from creeping in, a set of control experiments are included to ensure each stage of the test is working as planned.
The first stage is the extraction of nucleic acids from the patient’s specimen. To check this has been done well, a human specimen control sample is included in the test kit. Both of these samples are needed to be tested positive for the presence of a common human gene (RNase P gene).
Next, a sample of SARS-CoV-2 nucleic acid sequences are included in the test kit. They are used as positive controls. These control samples need to be tested positive to ensure the RT-qPCR is functioning properly. Additionally, a negative control is also done that contains no biological sample and that should give negative results to all the tests.
A unanimous confirmation is only given when a patient’s sample is tested positive for not one, but three segments of sequences that are specific to SARS-CoV-2 and all the control experiments showed their expected results.
Let us not forget to thank all the laboratory workers for their hard work in providing us with reliable screening tests! Should you feel unwell with even the slightest symptoms of COVID-19, you should arrange for a screening test at a private or government clinic at the earliest instance!