The Chemistry of Christmas Festivities
Dr. Jason Chan, Department of Chemistry, brings us lesson to a few of the molecules responsible for the scents of Christmas.
By Dr. Jason Chan, Department of Chemistry, HKUST
We are in the season of Advent, counting down towards Christmas. We may celebrate Christmas by decorating the house with a Christmas tree, having a few mince pies and a glass or two of mulled wine. In this article, let’s explore a few of the molecules responsible for the scents of Christmas.
Let’s start at the tree, a real Christmas tree (not the fake plastic ones) is a pine tree and it has a characteristic scent of pine – try to sniff a few needles of fresh pine next time you come across a real Christmas tree. The major scent molecules are α-pinene and β-pinene. As you may notice, these molecules were named after the tree.
Mulled wine is a warming sweet drink with a festive flavour. It is easy to prepare: just simmer some red wine with spices (usually cinnamon, cloves and star anise) and fresh oranges and sweetened with sugar to taste. The aroma is incredible: the hot alcoholic broth helps to release aroma molecules into the drink, such as cinnamaldehyde from cinnamon bark, eugenol from whole cloves, anethole from star anise and limonene from orange peels.
Delighted with the spiced drink, you reach for the mince pies, warm from the oven. Mince pies are traditionally served at Christmas time: the shape of the pies resemble the crib in the manger. The filling of mince pies is a mixture of meat, fruits and spices, typically cloves and nutmeg. The tones of nutmeg is particularly strong in mince pies and it is actually myristicin that we are smelling.
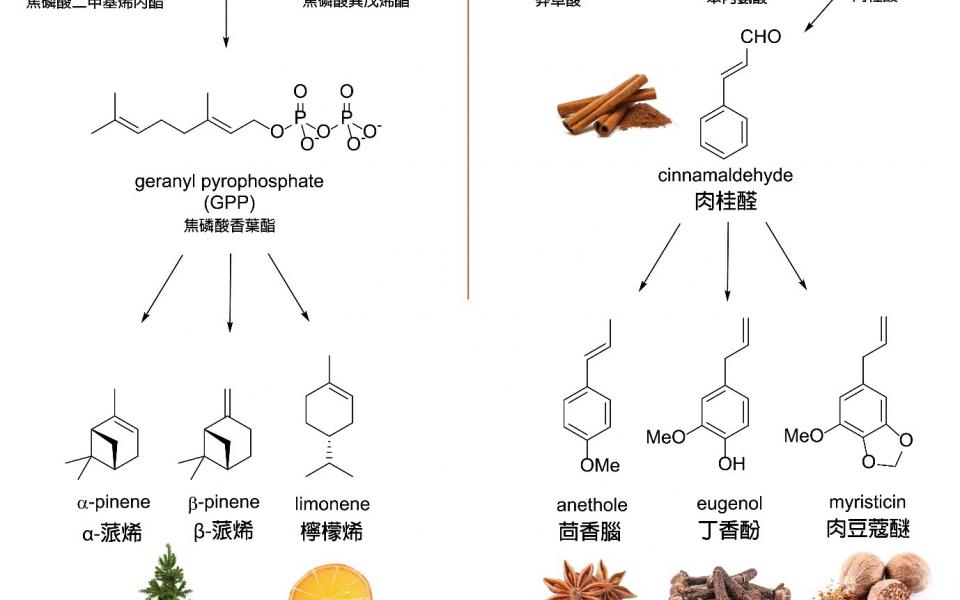
Seven aroma molecules associated with the festive season were highlighted above, and perhaps we can learn a lesson of biological chemistry by looking into how they were produced in the various plants. We can classify these seven compounds into two classes: monoterpenes and phenylpropanoids.
Monoterpenes come from the terpene pathway. It is simple to understand this pathway: there is one five-carbon starter unit (dimethylallyl pyrophosphate or DMAPP) which is extended by being joined to another five-carbon unit (isopentenyl pyrophosphate or IPP). Do this once, you get a ten-carbon unit called geranyl pyrophosphate or GPP. GPP can then be transformed into various monoterpenes, including α- and β-pinenes and limonene. You can keep adding IPP units, and thus end up with terpene molecules in multiples of 5-carbons (C10, C15, C20, etc.).
Phenylpropanoids come from the shikimate pathway. In this pathway, shikimate (an important metabolite intermediate in plants) is first converted into an aromatic amino acid phenylalanine. From phenylalanine, cinnamic acid is formed by elimination of ammonia. Then by reducing cinnamic acid, cinnamaldehyde is produced. Further reduction and modification of cinnamaldehyde gives eugenol, anethole and myristicin.
Just as the celebration of Christmas can be traced back to biblical accounts of the birth of Jesus, and further traced to events and prophecies in the Old Testament; the molecules that are found in a plant, animal or microorganism can also be traced back to their originating pathways and precursors. Unravelling the biosynthesis of natural products allows us to admire the intricate machines of Chemistry beneath the face of Biology!